First clinical studies about the application of the bio adhesive have been carried out!
Already for a continued period of time bio adhesive is applied in medicine as a treatment method but it is novelty in vein treatment! In the end of 2010 broader public learned more about the application of the biological adhesive in case of varicose veins, but in September, 2011, this Sapheon VenaSeal medical device were approved with the European CE marking*. In Latvia closing of veins with biological glue is available from 2013.
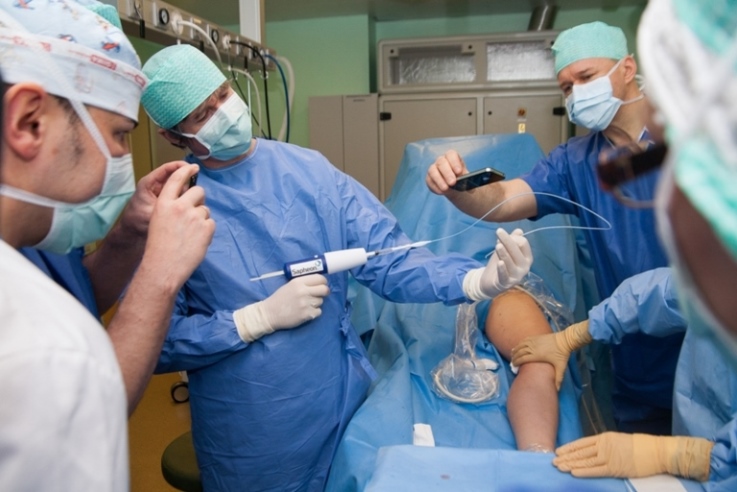
Endovenous heat ablation methods are safe and effective for the more severe cases of the chronic vein insufficiency. But vein surgeries with the usage of biological adhesive is especially recommended in the initial stage of vein diseases as they are very caring, without the application of heat and traumatic action onto the vein, surrounding tissues and nerves. In comparison to other surgeries (surgical where radio frequencies, laser, steam is applied), it does not require anaesthesia, compression socks are not needed after the surgery as there are no side effects – oedema, hematoma etc.
Besides first clinical studies have been performed approving effectiveness and safety of this technology.
* CE marking confirms that the product meets the basic requirements set by the European Union as regards to safety, health and environmental protection, and all necessary compliance evaluation procedures have been performed to this product.
You can read more about the biological adhesive method and results of the clinical studies here:

